R&D center
3SBio always regards product quality as the lifeline of the enterprise.
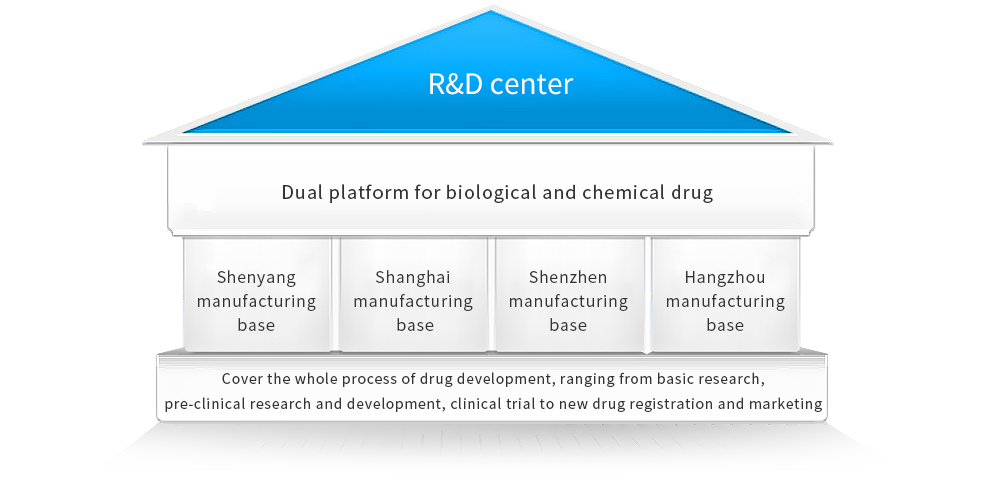
The Group has the National Engineering Research Center of Antibody Medicine approved by the National Development and Reform Commission. Members of its Academic Committee are all composed of authoritative academicians and well-known experts. We established 4 major R&D bases in Shenyang, Shanghai, Shenzhen and Hangzhou, successfully building the dual platform for biological and chemical drug, covering the whole process of drug development, ranging from basic research, pre-clinical study, and clinical trial to new drug registration and marketing.
R&D platform
We have three R&D platforms, including two protein-based biopharmaceutical platforms (a cellular platform for mammalian expression system and a platform for bacterial cell expression system) and a chemical platform.
R&D process
Our findings and pre-clinical studies involve the following general steps:
-

Identify and select molecules that have drug efficacy and market potential.
-

Include process development and control, structural verification, quality standards and stability studies. All of these studies are conducted in accordance with regulatory guidelines to demonstrate that product quality and manufacturing processes meet sufficiently high standards.
-

Analyze the efficacy and safety of the product under study on animal subjects to guide follow-up clinical trials.
We continue to research process development and control throughout the pre-clinical and clinical stages until the commercialization of the product. The R&D project is carried out by four departments within the company:
-
Research
institutionResearch
-
External cooperation
departmentcooperation
-
Clinical
departmentclinic
-
Registration affair
departmentregistration
- Research institution. The research institution specializes in the development of biopharmaceutical products expressed in mammalian cells and bacterial cells and is the main unit responsible for our internal pre-clinical R&D.
- External cooperation department. The external cooperation department mainly focuses on identifying companies that possess promising chemical and pharmaceutical products and monoclonal antibody drugs, and is the main unit responsible for cooperative pre-clinical R&D.
- Clinical department. The clinical department is responsible for designing and managing clinical trials.
- Registration affair department. The registration affair department is responsible for registering our products to the NMPA and supervising research and development projects to ensure compliance with relevant Chinese regulations in the development, registration, and commercialization of pharmaceuticals.